v-innovate Technologies, as the leader of bio-tech industry, devote to forge high quality targeted metabolomics service with advanced apparatus and professional research team. We provide high throughput, high precision and short time-consuming Heparan Sulfate/Heparin Analysis Service, which could meet all the needs of our customers, to assist your scientific research.
Heparin (HP)/heparan sulfate (HS), a polyanion and heteropolysaccharide, belongs to the glycosaminoglycan family. HP/HS is ubiquitously present on the cell surface and extracellular matrix (ECM), as well as in the intracellular environment (mast cells). Since its discovery in 1916, HP and its low-molecular preparations, one of the most important anticoagulants, have been widely used in clinical treatment. The various structural features of HP/HS allow these molecules to interact with various protein (e. g., enzyme inhibitor, chemokine, growth factor, morphogen and other signaling proteins) to participate in various physiological and pathological processes such as coagulation, cell adhesion, inflammation, cell migration, differentiation, and even pathogen infection. These important biological effects have aroused great attention in the structural and functional studies and clinical applications of HP/HS.
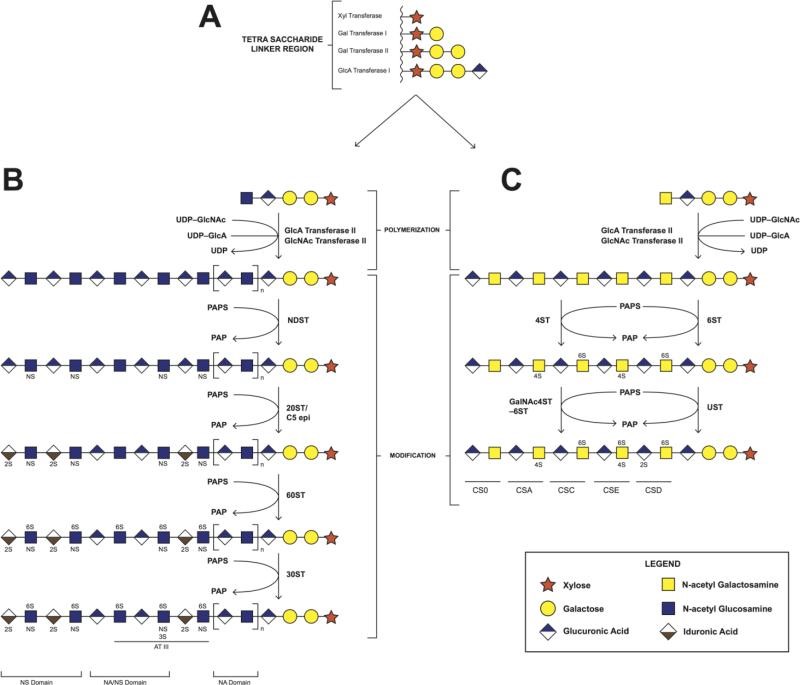 Fig 1. Biosynthesis of heparin/heparan sulfate and chondroitin sulfate. A. synthesis of tetrasaccharide linker region; B. the polymerization and modification pathway of heparin/heparan sulfate; C. the polymerization and modification pathway of chondroitin sulfate.
Fig 1. Biosynthesis of heparin/heparan sulfate and chondroitin sulfate. A. synthesis of tetrasaccharide linker region; B. the polymerization and modification pathway of heparin/heparan sulfate; C. the polymerization and modification pathway of chondroitin sulfate.
The backbone of HP/HS polysaccharides is composed of repeating disaccharide units consisting of D-glucuronic acid (GlcA)/L-iduronate acid (IdoA) and N-acetyl-D-glucosamine (GlcNAc). During the biosynthesis of HP/HS, their repeated disaccharides, which initial the synthesis of a common precursor consisting of the units of GlcA-GlcNAc, followed by further modification by several enzymes, including N-deacetylase-N-sulfo and 3- and 6-sulfo, which modify GlcNAc residues; A C5- epimerase that converts GlcA to an IdoA residue; And 2-O- sulfotransferase to catalyze the sulfation of GlcA/IdoA residues. These modifications result in variation of disaccharide in HP/HS polysaccharides, making HP/HS the most complex polymer in nature. Therefore, the high structural complexity seriously hinders the structural and functional research of HP/HS.
To date, three types of liver enzymes have been identified based on their substrate specificity: Liver enzyme I(EC4.2.2.7), which specifically degrades highly sulfated and IdoA-rich HP; HE Pase III (EC 4.2.2.8), which tends to degrade low sulfate and GlcA-rich HS; And hepae II (EC 4.2.2.-), which digest HP and HS. Structurally, Hepase I fold into a beta-jelly-roll structure and tend to cleave that α-1,4-bond attached to the IdoA2S/GlcA2S residue. In contrast, Hepase III consists of an N-terminal (α/α)5-barrel domain and a C-terminal antiparallel β-sandwich domain, and prefers to cleave the α-1,4- link to residue of GlcA/IdoA. Hepase II employs a topology similar to that of Hepase III, and has structures with no selectivity for, in some cases, GlcA/IDOA or IdoA2S/GlcA2S. All Hepases are shared, with His- tyrosine serving as a similar catalytic mechanism for bronsted bases and acids. It should be noted that all the heparinases identified belong to the endonuclease family, which randomly cleaves the internal HP/HS chain, while exocrine heparanase has only been discovered so far as a very useful tool for sequencing the HP/HS chain.
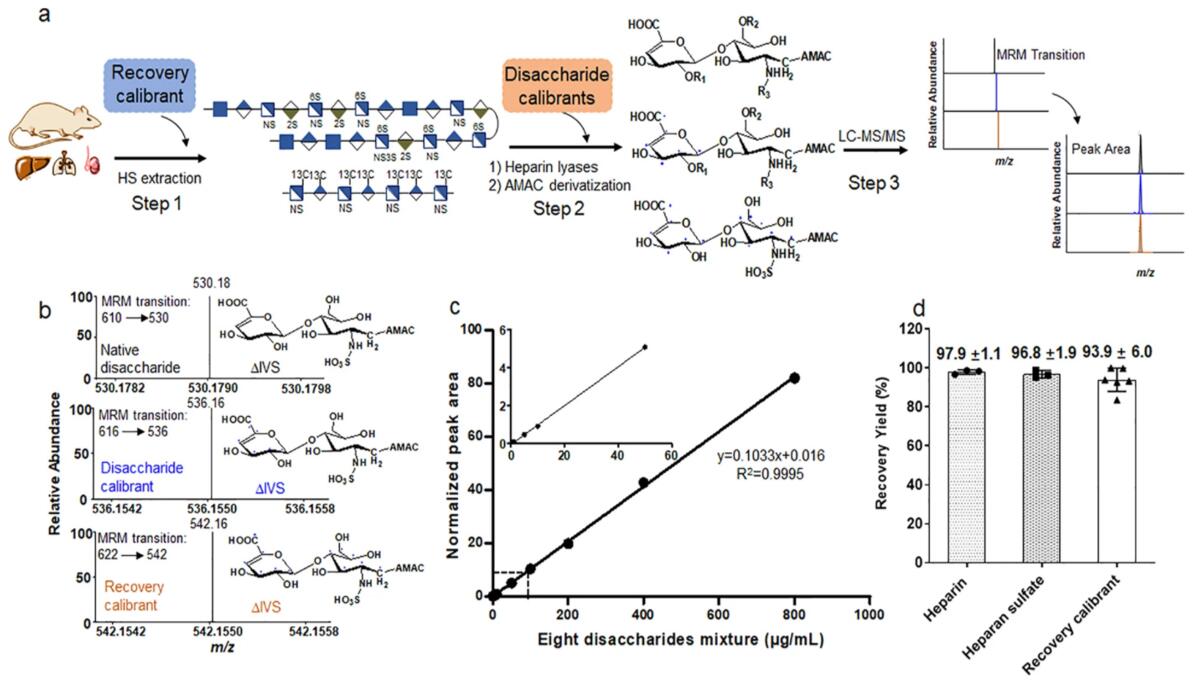 Fig 2. Figure A shows a work-flow for performing disaccharide composition analysis with three steps. The recover calibrators are added in that heparan sulfate extraction stage or step 1; A disaccharide calibrator is added in the heparin lyase digestion stage or step 2; A disaccharide calibrator was mixed with various concentrations of unlabeled disaccharide and analyzed by LC-MS/MS in analysis stage or step3. Figure B shows the ion chromatograms of the three isotope-labeled formats disaccharide from MRM. Figure C shows the dynamic range of analysis in the presence of a disaccharide calibrator.
Fig 2. Figure A shows a work-flow for performing disaccharide composition analysis with three steps. The recover calibrators are added in that heparan sulfate extraction stage or step 1; A disaccharide calibrator is added in the heparin lyase digestion stage or step 2; A disaccharide calibrator was mixed with various concentrations of unlabeled disaccharide and analyzed by LC-MS/MS in analysis stage or step3. Figure B shows the ion chromatograms of the three isotope-labeled formats disaccharide from MRM. Figure C shows the dynamic range of analysis in the presence of a disaccharide calibrator.
Repeated freezing and thawing of samples must be avoided. The serum sample should be precipitated in the collection tube for 30 minutes at room temperature, then transported to the centrifuge tube and centrifuged at 8000 rpm for 5 minutes. After centrifugation, the supernatant was equally divided into a freezing tube of 500 uL / sample.
Anticoagulants and preservatives must be added immediately after collection and then frozen at -80 °C.
Urine samples should be equally divided into centrifuge tubes with 1 mL per tube, each tube is added with 1/100 (w/v) sodium azide and stored at -80 °C.
Samples should be frozen in liquid nitrogen immediately and then transported to -80 °C for storage after collected.
Cytoactive should be terminated immediately to maintaining cell integrity.
In general, to assure enough sample to fulfill the whole project, the volume of the single sample need to be offered as much as possible. The remaining samples will be stored for one year free of charge and returned at any time if necessary. All samples need to be stored and transported at -80°C and try to avoid using surfactants (SDS, Triton-X) and inorganic salts.
Clinical samples are repeated in no less than 30 cases in a single group.
Animal samples are repeated in no less than 9 cases in a single group.
v-innovate Technologies metabolism analysis platform is committed to the all-around, reliable and accurate analysis service for a variety of target substances, which is suitable for life-science research, drug exploration, biological determination and other fields. We sincerely hope to cooperate with you to assistant your scientific research.
References
